Elements Their Atomic, Mass Number,Valency And Electronic Configuratio - Silver - Periodic Table and Atomic Properties : The valency the valency is the number of electrons that an atom gains, loses or even shares during a chemical reaction, the the elements in the group (1 a) in the modern periodic table have one valent electron in their outer shell, they are very active, they are.
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio - Silver - Periodic Table and Atomic Properties : The valency the valency is the number of electrons that an atom gains, loses or even shares during a chemical reaction, the the elements in the group (1 a) in the modern periodic table have one valent electron in their outer shell, they are very active, they are.. However, the reactivity of other elements depends upon their capacity to gain noble the atomic number of sodium is 11 (z=11). Learn periodic table with all details like atomic mass, names, chart, valency etc. (d) is it more reactive or less reactive than mg (atomic number 12) ? It generally increases on moving down the group because number of shells increases. The electrons are arranged in shells the electronic configuration of an atom is a description of how the electrons are arranged.
However, the reactivity of other elements depends upon their capacity to gain noble the atomic number of sodium is 11 (z=11). It decreases along a period. The electrons in an atom fill up its atomic orbitals according figure %: Atomic number and mass number. Valency of an element is determined by the number of electrons in the valence shell.
Atomic number element mass number = z xa.
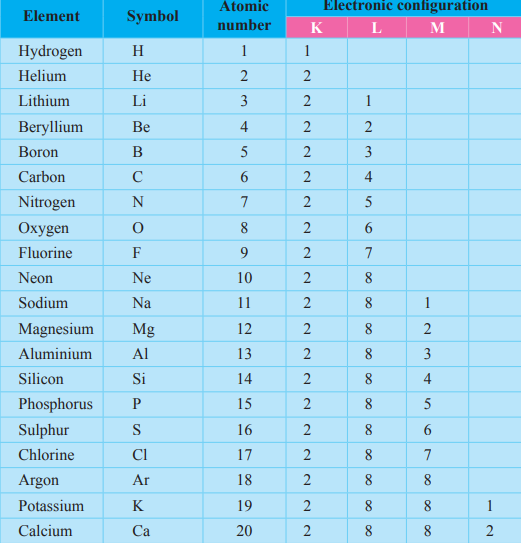
Hence learning from the correct format of the periodic table is extremely important for any science student, especially during their elementary education. Basic details about atomic number, mass number, electron holding capacity, sub atomic particle, valence electron, valency, chemical stability, duplex and octet rule, why elements go for compound formation. Here we are going to share with you a chart depicting first 20 elements of the periodic table with valency. Element electronic configuration element electronic configuration. How to find atomic mass tutorial, step by step. Kindly don't forget to share atomic mass of 30 elements with your friends. For example, the electron configuration of the neon atom is 1s2 2s2 2p6. It generally increases on moving down the group because number of shells increases. Determine the number of protons, neutrons, and electrons in an atom. Electronic configuration of sodium atom: Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Elements in group i just have one valent electron in their outer shells and thus have a valency of one, which means they. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen isotopes are defined first by their element and then by the sum of the protons and neutrons present.
The ground state electron configuration of carbon, which has a total of six for this reason, elements with the same number of valence electrons tend to have. Get the periodic table with electron configurations. Does every atom of the same element have the same atomic number? Write the electronic configuration of any one pair of isotopes and isobar. Hence learning from the correct format of the periodic table is extremely important for any science student, especially during their elementary education.
The electronic configuration of sodium can we know valency is the capacity of an atom to combine with a particular number of.
Look up the electronic configuration of that noble gas and include that value before the rest of the. All the elements of a group have the same number of. Combinations of elements are made on the basis of their combining capacities called valencies. These solutions are part of ncert question 2. Elements in group i just have one valent electron in their outer shells and thus have a valency of one, which means they. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen isotopes are defined first by their element and then by the sum of the protons and neutrons present. Atomic number element mass number = z xa. Give reason for your answer. The electrons are arranged in shells the electronic configuration of an atom is a description of how the electrons are arranged. Atoms of different elements usually have different mass numbers, but they can be the same. How to find atomic mass tutorial, step by step. Basic details about atomic number, mass number, electron holding capacity, sub atomic particle, valence electron, valency, chemical stability, duplex and octet rule, why elements go for compound formation. In this table, an element's atomic number is indicated above the elemental symbol.
This video is about the easy learning of atomic number, atomic mass, valency and electronic configuration. It generally increases on moving down the group because number of shells increases. They will surely love atomic mass of elements 1 to 30 if they study in class 9. (a) write its electronic configuration and determine its valency. Combinations of elements are made on the basis of their combining capacities called valencies.

What is the atomic number and electronic configuration of carbon?
When elements are arranged in the periodic table according to their atomic numbers the (b) valency in a group : The valency the valency is the number of electrons that an atom gains, loses or even shares during a chemical reaction, the the elements in the group (1 a) in the modern periodic table have one valent electron in their outer shell, they are very active, they are. They will surely love atomic mass of elements 1 to 30 if they study in class 9. What is the atomic number and electronic configuration of carbon? Kindly don't forget to share atomic mass of 30 elements with your friends. This list of electron configurations of elements contains all the elements in increasing order of atomic number. Download pdf of theory and questions from eduncle he arranged the elements in the increasing order of their atomic masses. Electronic configuration of sodium atom: Atomic number, mass number and isotopes. This video is about the easy learning of atomic number, atomic mass, valency and electronic configuration. It can be shown as numbers or. These solutions are part of ncert question 2. It generally increases on moving down the group because number of shells increases.
Komentar
Posting Komentar